Capturing the antimicrobial profile of Paeonia officinalis, Jasminum officinale and Rosa damascene against methicillin resistant Staphylococcus aureus with metabolomics analysis and network pharmacology
In the current study, published in scientific reports journal, we evaluated the in vitro antibacterial efficacy of the roots’ extracts of Jasminum officinale, Rosa damascene and Paeonia officinalis against MRSA (methicillin-resistant Staphylococcus aureus) by well diffusion technique. The root extract of P. officinalis exerted a potent anti-MRSA with MIC 0.4673 µg/ml, while both J. officinale and R. damascene exhibited very weak activity. The dried roots of each P. officinalis, J. officinale and R. damascene (650 g of each) were macerated separately in 95% methanol at room temperature until exhausted. After that, reduced pressure was used to get rid of the alcohol, affording a viscous syrupy residues (60, 72, 65 g) for P. officinalis, J. officinale and R. damascene consequently. Therefore, chemical profiling of the crude extract P. officinalis roots assisted by LC-HR-ESI-MS was performed and led to the dereplication of twenty metabolites of different classes, in which terpenes are the most abundant compounds. On a molecular level, network pharmacology was used to determine the targets of active metabolites to bacterial infections, particularly MRSA. Online databases PubChem, UniProt, STRING, and Swiss Target Prediction were used. In addition to using CYTOSCAPE software to display and analyze the findings, ShinyGO and FunRich tools were used to identify the gene enrichment analysis to the set of recognized genes. The results detected the identified metabolites were annotated by 254 targets. ALB, ACHE, TYMS, PRKCD, PLG, MMP9, MMP2, ERN1, EDNRA, BRD4 were found to be associated with MRSA infection. The top KEGG pathway was the vascular smooth muscle contraction pathway according to enrichment FDR. The present study suggested a possible implication of P. officinalis roots as a potent candidate having a powerful antibacterial activity against MRSA.
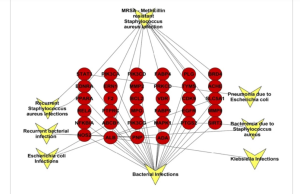
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the major causes of hospital- and community-associated infections. MRSA infections have the capability to resist the impacts of many common antibiotics such as methicillin, penicillin and other common antibiotics. MRSA infections are more complicated to be treated. Moreover, can influence the bloodstream, heart, bones, lungs, and joints of MRSA casualties, and can transmit from an object which contains MRSA to a human, or from a human carrier to another human. Staphylococcus aureus can cause various infections, ranging from common skin and respiratory problems to life-threatening conditions like necrotizing fasciitis and a severe form of pneumonia (necrotizing pneumonia). MRSA can cause different types of infections like Healthcare associated MRSA, Community associated MRSA and Livestock associated MRSA infections. Natural products exerts a great role in MRSA prevention, the herbals extracts of Garcinia mangostana and Quercus infectoria have considerable activity against MRSA. Furthermore, curcumin, garlic, ginger, Thai longan honey, Juncus and Luzula species, Greek oregano, Baru plant, and Lichen are natural products that showed great potential against drug-resistant S. aureus. The usage of natural products in therapeutic management against MRSA caused diseases have a benefit over the application of synthetic drugs due to the decreased side effects of natural products extracts.
The antimicrobial activity examination of the crude extracts of three roots; J. officinale, R. damascene and P. officinalis roots against MRSA were performed via the well-diffusion assays revealing the highest antibacterial potency of P. officinalis extract against MRSA with MIC 0.4673 µg/ml, while the other two extracts showed no activity (> 100 µg/ml). The MIC for ciprofloxacin against MRSA was 19.2 µg/ml. Peony plants and their metabolites are well known with their varied biological activities including; anti-inflammatory, immunomodulatory, neuroprotective, antiviral, antidiabetic, etc. HR-LC-ESI-MS analysis of the crude P. officinalis extract was performed for highlighting the metabolites responsible for the strong anti-MRSA potency.
254 target genes were found to be targeted by the identified metabolites the p. officinalis, a network (metabolites–targets) was formed, the network composed of 285 nodes and 683 edges, with network centralization 0.289, no targets were found for compounds 7, 10, 13, the formed network identified CDA, ADA, ADK and ADORA2A genes as the top targets related to the metabolites with 9 edges for each.
We utilised FunRich software to perform a gene ontology and enrichment analysis on all the active metabolites' targets to determine the cellular components, molecular functions, and biological processes that are affected by this group of genes. The results confirmed that signal transduction, metabolism and the energy pathway are the most prominent biological processes, in that order. The Cytoplasm, the Nucleus and the Plasma membrane are the top three cellular component. Catalytic activity was the top molecular function, followed by G-protein coupled receptor activity and protein serine/threonine kinase activity. Using an enrichment analysis implemented in ShinyGO v0.741, we were able to identify the most significant biological pathways associated with the target genes; these pathways include the vascular smooth muscle contraction pathway was the top KEGG. pathway according to enrichment FDR (Fold Discovery Rate) followed by proteoglycans in cancer and calcium signaling pathway
The molecular docking approach was employed to investigate the binding affinities and potential interactions of the compounds with the target proteins. The twenty compounds were subjected to molecular docking analysis against possible potential target proteins encoded by the genes ACHE, TYMS, PRKCD, MMP9, MMP2, ERN1 and BRD4. The crystal structures of these targets were selected from the Protein Data Bank (PDB), including acetylcholine esterase (PDB ID: 4EY7), (PDB ID: 6QXG), protein kinase C delta (PDB ID: 1PTR), matrix metalloprotease 9 (MMP9) (PDB ID: 1GKC), MMP2 (PDB ID: 1HOV), inositol requiring enzyme 1 (IRE1) (PDB ID: 4U6R), and Bromodomain-containing protein 4 (PDB ID: 3MXF). Previous studies explained the importance of the catalytic triad residues Ser203, Glu334 and His447 in the AChE active site45. Docking studies revealed that Ser203, Glu202, and His447 amino acids stabilize the compound 16 in the active site of AchE (Fig. 9A). Moreover, compound 16 was able to bind significantly with protein kinase C by H-bonding with Gly253 and Leu251. For thymidylate synthase (PDB ID: 6QXG), the co-crystallized ligand showed H-bonding interactions of ASP 218 with a C=O group and ASN 226 with N–H and a C=O group at the 3 and 4 positions. The binding pattern of compounds 14 was found to be similar to the thymidylate synthase protein with Asn226 and His256. These interactions contribute to the stability and specificity of the compound's binding.
he anti-MRSA activity of three root extracts of three medicinal plants J. officinale, R. damascene and P. officinalis roots was evaluated and revealed the strongest potency of P. officinalis roots. This is the first study of the antimicrobial evaluation of P. officinalis roots, therefore, untargeted metabolomics profiling of the root derived extract of P. officinalis was performed by using LC-HR-ESI-MS, in which twenty compounds of multivariate groups of secondary metabolites like terpenoids, terpenidal glycosides and phenolic compounds were dereplicated. The analysis of the molecular docking data provides valuable insights into the binding affinities and potential specificities of the 20 compounds against the 7 biological targets. Future experiments will include isolation of the bioactive compounds and test them against MSRA as well as study the detailed mechanism of action in vitro as well as in vivo. These findings can guide further investigations and contribute to the rational design and development of novel therapeutics or lead compounds in drug discovery research.
